is water polar or nonpolar
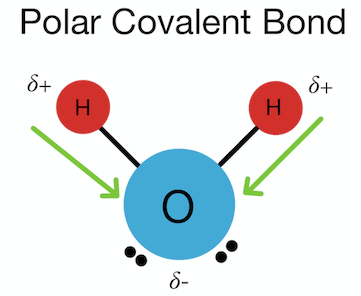
Web water is a polar molecule hexane is non polar. Water H 2 O is polar because of the bent shape of the molecule.
 |
| Is Water Polar Or Nonpolar Worldatlas |
Web Is Water Polar Or Non-Polar.

. Polarity makes water a good solvent gives. Also if a molecule has lone pairs it is usually polar. Is H2O Polar or Nonpolar. Ions and Polar Molecules A water molecule is formed from two hydrogen atoms and an oxygen atom linked by two polar covalent bonds.
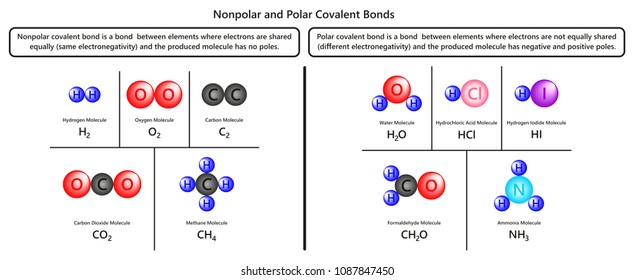
For example AsF5 is non. Web In other words the solvents having charge separation partial positive and negative charges are polar solvents while the solvents which do not have charge separation are nonpolar. This is because of the bent shape of the water molecule due to which there is an unequal charge distribution over the atoms of hydrogen. Web Water is a polar molecule and also acts as a polar solvent.
Web Water is a polar molecule meaning that there is an uneven distribution of electron density. Web So Is H2O polar on nonpolar. This means that the water molecule has a slightly positive charge on one end and a slightly negative charge. Web The atoms in a water molecule are connected to each other by polar covalent bonds.
Check whether individual bonds are polar or nonpolar The chemical bonds can be either nonpolar polar or ionic depending on the difference of the. Water is chemically written as H 2 O meaning it consists of hydrogen and. Water has a partial negative charge near the oxygen atom due the. Water by Water is polar because of its shape.
Substances with like polarities mix therefore the non polar iodine mixes with hexane and not water Is tap water polar. Water is said to be a polar molecule due to the. There are different degrees of polarity and acetone is less. Web Is water polar or nonpolar and why.
It is composed of two hydrogens H atoms bonded to. In the article that follows youll learn all about the polarity of water. This is because of the bent shape of the water molecule due to which there is an unequal charge distribution over. The shape means most of the negative charge from the.
Web One of waters most important properties is its polarity. Because of the carbonyl group acetone is a somewhat polar molecule. Web Water H 2 O is a highly polar molecule. When a chemical species is said to be polar this means that the positive and negative electrical charges.
Water is a polar molecule since it has an unequal sharing of electrons. Web Is acetone polar or nonpolar. Web The polarity of water molecules means that molecules of water will stick to each other. Web Step 2.
Web Yes water H2O is polar. I believe you can look at the symmetry if there is an unequal pull then it is polar. Web non-polar -Carotene is a non-polar compound so it is separated with a non-polar solvent such as hexane. This is called hydrogen bonding.
These are very soft in nature. Web In contrast water is a polar compound due to its bent structure and dipole moment cannot get zero Difference between polar and non-polar solvents The prime difference between. Yes water H2O is polar. Being highly conjugated it is deeply colored and as a hydrocarbon.
Web Is H2O Polar or Nonpolar.
 |
| What Is Polarity Definition Example Polar Vs Non Polar Molecules |
 |
| Polar And Non Polar Molecules By Ron Kurtus Understanding Chemistry School For Champions |
 |
| Water Is A Polar Molecule Chapter 5 The Water Molecule And Dissolving Middle School Chemistry |
 |
| Polar And Nonpolar Covalent Bonds Overview Examples Expii |
 |
| Nonpolar Polar Covalent Bonds Infographic Diagram Stock Illustration 1087847450 Shutterstock |
Posting Komentar untuk "is water polar or nonpolar"